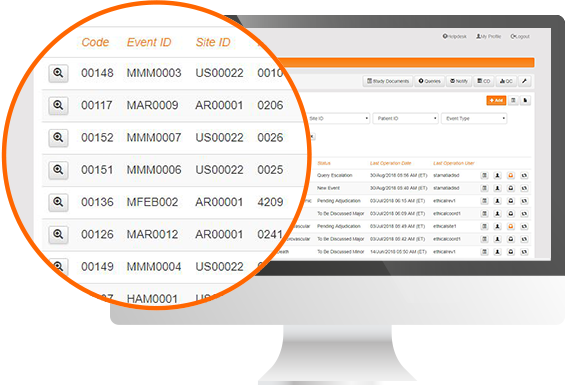
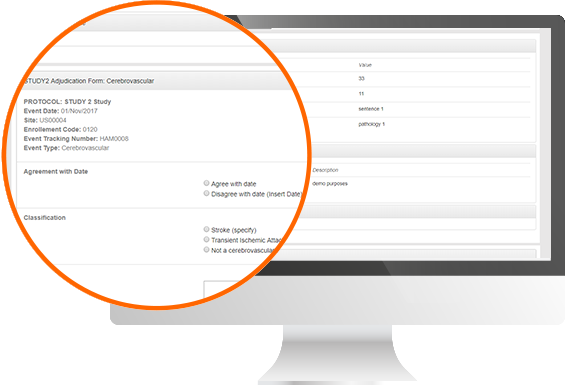
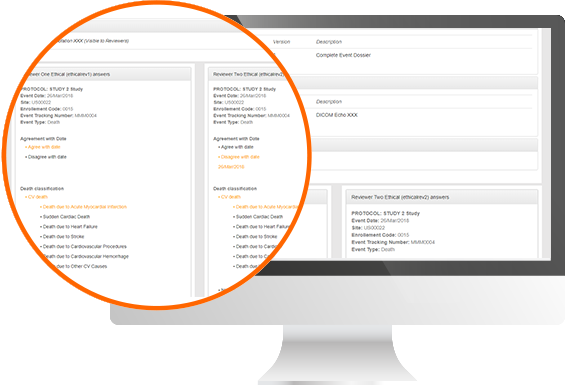
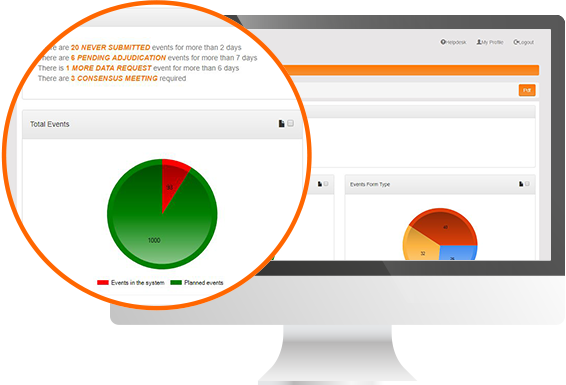
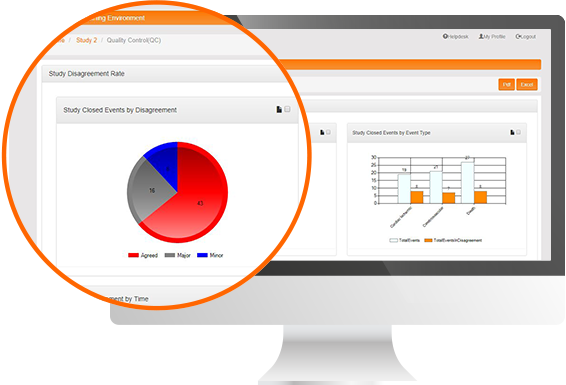
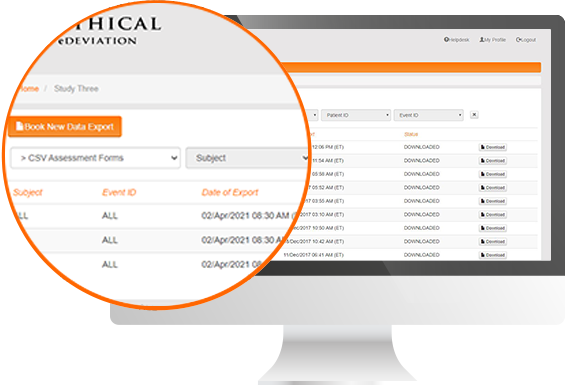
Protocol Deviation
Open/Close sub menu
 CRO-Safe
CRO-Safe
Partnership Program Ethical helps CROs stay ahead of competition READ MORE GxP Compliance Open/Close sub menu
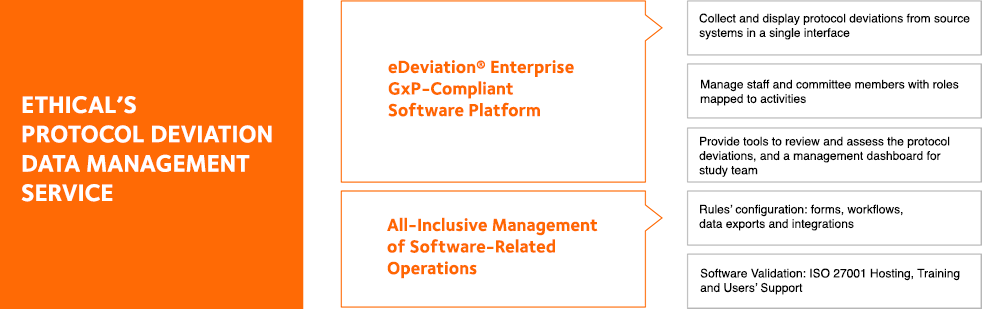
PROTOCOL DEVIATION KNOWLEDGE
Protocol Deviation RegulationsProtocol Deviation GlossaryProtocol Deviation HandbookProtocol Deviation ResourcesOUR EXPERTISE
Endpoint AdjudicationSAE ReconciliationData Safety Monitoring Boards eDeviation Software Open/Close sub menuSOFTWARE SOLUTION
eDeviation Software VideoWHO WE HELP
CRO-Safe Partnership Program CRO-Safe
CRO-SafePartnership Program Ethical helps CROs stay ahead of competition READ MORE GxP Compliance Open/Close sub menu